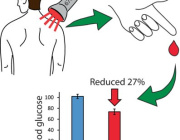
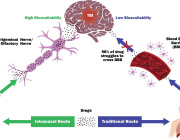
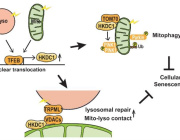
Noninvasive Treatment of Brain Ischemia-Reperfusion Injury With Near Infrared Light: Working Toward Clinical Implementation
 Dr. Maik Hüttemann, from Wayne State University, USA, will join us this year to present his most recent findings on "Noninvasive Treatment of Brain Ischemia-Reperfusion Injury With Near Infrared Light: Working Toward Clinical Implementation" within a session, chaired by him, entitled "Translational Therapies - Focus on Infrared Therapies".
Dr. Maik Hüttemann, from Wayne State University, USA, will join us this year to present his most recent findings on "Noninvasive Treatment of Brain Ischemia-Reperfusion Injury With Near Infrared Light: Working Toward Clinical Implementation" within a session, chaired by him, entitled "Translational Therapies - Focus on Infrared Therapies".
Dr. Hüttemann has discovered specific wavelengths of near infrared light (IRL) that target and partially inhibit the mitochondrial enzyme cytochrome c oxidase (COX). Modulation of COX activity via IRL allows him to control the electron transport chain (ETC) and would be therapeutically beneficial in conditions where mitochondria are hyperactive, such as during reperfusion following an ischemic event. ETC hyperactivity is detrimental to neurons because it causes hyperpolarization of the mitochondrial membrane potential during reperfusion, leading to the generation of excessive amounts of reactive oxygen species (ROS).
Dr. Hüttemann shows that noninvasive application of COX-inhibitory IRL in small and large animal models of brain ischemia/reperfusion injury limits ROS production and is highly neuroprotective. To translate these findings into the clinic, efficient and safe IRL delivery to the human head is essential, which is achieved through novel silicone based IRL delivery patches.
Join us in Targeting Mitochondria 2022 and benefit from the experience of professional researchers in this field. Book your spot.
Targeting Mitochondria 2022 Congress
October 26-28, 2022 - Berlin, Germany
wms-site.com
A Non-canonical Tricarboxylic Acid Cycle Underlies Cellular Identity
 Dr. Paige Arnold, from the Memorial Sloan Kettering Cancer Center, USA, will join us this year to present her most recent findings on Tricarboxylic acid cycle, through a presentation entitled "A Non-canonical Tricarboxylic Acid Cycle Underlies Cellular Identity".
Dr. Paige Arnold, from the Memorial Sloan Kettering Cancer Center, USA, will join us this year to present her most recent findings on Tricarboxylic acid cycle, through a presentation entitled "A Non-canonical Tricarboxylic Acid Cycle Underlies Cellular Identity".
Dr. Arnold states that the tricarboxylic acid (TCA) cycle is a core metabolic pathway that produces reducing equivalents for energy production and critical biosynthetic intermediates. Despite the ubiquitous importance of TCA cycle-derived products for cell viability and proliferation, mammalian cells display significant heterogeneity in TCA cycle activity. The existence of diverse TCA cycle wiring across different cell types led her and her team to hypothesize that TCA cycle enzymes might assemble into multiple pathway configurations. By combining genetic co-essentiality mapping with isotope tracing studies in cancer cells and stem cells, they describe a biochemical alternative to the traditional TCA cycle that is cell-state dependent and required for cell fate transitions.
Join us in Targeting Mitochondria 2022 and benefit from the experience of professional researchers in this field.Book your spot.
Targeting Mitochondria 2022 Congress
October 26-28, 2022 - Berlin, Germany
wms-site.com
Mitochondria Transplantation Between Living Cells
 Dr. Julia A. Vorholt, from ETH Zurich, Switzerland, will join us this year to present her most recent findings on "Mitochondrial Transplantation Between Living Cells" in a session entitled "Mitochondria Transplantation and Transfer"
Dr. Julia A. Vorholt, from ETH Zurich, Switzerland, will join us this year to present her most recent findings on "Mitochondrial Transplantation Between Living Cells" in a session entitled "Mitochondria Transplantation and Transfer"
Mitochondria and the complex endomembrane system are hallmarks of eukaryotic cells. To date, it has been difficult to manipulate organelle structures within single live cells.
Dr. Vorholt developed a FluidFM-based approach to extract, inject and transplant organelles directly from and into living cells with subcellular spatial resolution. The technology combines atomic force microscopy, optical microscopy and nanofluidics to achieve force and volume control with real-time inspection.
The approach enables the transfer of controlled quantities of mitochondria into cells while maintaining their viability and monitoring their fate in new host cells. Transplantation of healthy and drug-impaired mitochondria into primary keratinocytes allows real-time tracking of mitochondrial subpopulation rescue. After transfer of mitochondria into somatic cells and cell propagation over generations, she shows that donor mtDNA was replicated in recipient cells without the need for selection pressure.
The approach opens new prospects for the study of organelle physiology and homeostasis, but also for mechanobiology, synthetic biology, and therapy.
Remember that Targeting Mitochondria 2022 also gives you the chance to present your new research related to this session by submitting your abstract.
Targeting Mitochondria 2022 Congress
October 26-28, 2022 - Berlin, Germany
wms-site.com
Regulation of Mitochondrial Signaling in Cardiomyopathy During Sepsis
 Dr. Qun Sophia Zang, from Loyola University Chicago Health Science Campus, USA, will join us this year to present her most recent findings on "Regulation of Mitochondrial Signaling in Cardiomyopathy During Sepsis".
Dr. Qun Sophia Zang, from Loyola University Chicago Health Science Campus, USA, will join us this year to present her most recent findings on "Regulation of Mitochondrial Signaling in Cardiomyopathy During Sepsis".
The research objective in Dr. Zang’s laboratory is to understand the mechanisms underlying sepsis-induced cardiomyopathy and to identify potential new drug targets for this devastating critical care condition.
In particular, Dr. Zang is interested in how injured mitochondria and related production of mitochondria-derived danger-associated molecular patterns (DAMPs) incite myocardial inflammation, elevate oxidative stress, and cause cardiac dysfunction in sepsis.
She recently discovered the importance of a previously unidentified function of autophagyfactor Beclin-1 in the regulation of mitochondrial quality control in the heart during sepsis.
Her current investigations are designed to decipher the signal transduction of Beclin-1 in the control of cardiacmitochondrial homeostasis via mitophagy and mitochondria-associated membranes in septicsubjects using both genetic and pharmacological approaches.
Remember that you too can join us and present your latest research on the mitochondria by submitting your abstract.
Targeting Mitochondria 2022 Congress
October 26-28, 2022 - Berlin, Germany
wms-site.com
Sonlicromanol in Primary Mitochondrial Disease MELAS Spectrum Disorders
 Dr. Jan Smeitink, from Khondrion B.V., Netherlands, will join us this year to present his most recent findings on "Sonlicromanol in Primary Mitochondrial Disease MELAS Spectrum Disorders" .
Dr. Jan Smeitink, from Khondrion B.V., Netherlands, will join us this year to present his most recent findings on "Sonlicromanol in Primary Mitochondrial Disease MELAS Spectrum Disorders" .
MELAS spectrum disorders caused by the 3243A>G mutation in the MT-TL1 gene lead to hampered oxidative phosphorylation with, as immediate cellular consequences, both reductive and oxidative stress.The clinical spectrum is heterogeneous with classical mitochondrial encephalopathy, lactic acidosis and stroke-like episodes (MELAS) and maternally inherited diabetes mellitus and deafness (MIDD) as most frequently encountered phenotypes. Sonlicromanol modulates key metabolic and inflammatory pathways in the pathogenesis of MELAS-SD models.
Here, Dr. Smeitnik reports the results of preclinical and phase 1 and 2 clinical studies which all together will guide the design of the upcoming pivotal phase 3 KHENERFIN trial.
Targeting Mitochondria 2022 Congress
October 26-28, 2022 - Berlin, Germany
wms-site.com
The Role of Extracellular Vesicles in Mitochondrial Quality Control Mechanisms
 Dr. Marc Germain, from Université du Québec à Trois-Rivières, Canada, will join us this year to present his most recent findings on "The Role of Extracellular Vesicles in Mitochondrial Quality Control Mechanisms" in a session dedicated to "Extracellular Vesicles & Mitochondria: The Target".
Dr. Marc Germain, from Université du Québec à Trois-Rivières, Canada, will join us this year to present his most recent findings on "The Role of Extracellular Vesicles in Mitochondrial Quality Control Mechanisms" in a session dedicated to "Extracellular Vesicles & Mitochondria: The Target".
Most cells constitutively secrete mitochondrial DNA and proteins in extracellular vesicles (EVs). Dr. Germain, will explain how cells actively prevent the packaging of pro-inflammatory, oxidized mitochondrial proteins that would act as damage-associated molecular patterns (DAMPs) into EVs. He will describe the selectively targeted pathways responsible for distinction between material to be included into EVs and damaged mitochondrial content to be excluded.
Targeting Mitochondria 2022 Congress
October 26-28, 2022 - Berlin, Germany
wms-site.com
Mitochondrial, exosomal miR137-COX6A2 in schizophrenia
 Dr. Ines Khadimallah, from Lausanne University Hospital, Switzerland, will join us this year to present her most recent findings on "Mitochondrial, exosomal miR137-COX6A2 in schizophrenia".
Dr. Ines Khadimallah, from Lausanne University Hospital, Switzerland, will join us this year to present her most recent findings on "Mitochondrial, exosomal miR137-COX6A2 in schizophrenia".
Dr. Khadimallah will be discussing the following:
Early detection and intervention in schizophrenia requires mechanism-based biomarkers that capture neural circuitry dysfunction, allowing better patient stratification, monitoring of disease progression and treatment.
In prefrontal cortex and blood of redox dysregulated mice, oxidative stress induces miR-137 upregulation, leading to decreased COX6A2 and mitophagy markers (NIX, Fundc1, and LC3B) and to accumulation of damaged mitochondria, further exacerbating oxidative stress and parvalbumin interneurons (PVI) impairment. MitoQ, a mitochondria-targeted antioxidant, rescued all these processes.
Translating to early psychosis patients (EPP), blood exosomal miR-137 increases and COX6A2 decreases, combined with mitophagy markers alterations, suggest that observations made centrally and peripherally in animal model were reflected in patients' blood extracellular vesicles. Higher exosomal miR-137 and lower COX6A2 levels were associated with a reduction of ASSR gamma oscillations in EEG.
As ASSR requires proper PVI-related networks, alterations in miR-137/COX6A2 plasma exosome levels may represent a proxy marker of PVI cortical microcircuit impairment. EPP can be stratified in two subgroups: (a) a patients' group with mitochondrial dysfunction "Psy-D", having high miR-137 and low COX6A2 levels in exosomes, and (b) a "Psy-ND" subgroup with no/low mitochondrial impairment, including patients having miR-137 and COX6A2 levels in the range of controls. Psy-D patients exhibited more impaired ASSR responses in association with worse psychopathological status, neurocognitive performance, and global and social functioning, suggesting that impairment of PVI mitochondria leads to more severe disease profiles.
This stratification would allow, with high selectivity and specificity, the selection of patients for treatments targeting brain mitochondria dysregulation and capture the clinical and functional efficacy of future clinical trials.
Targeting Mitochondria 2022 Congress
October 26-28, 2022 - Berlin, Germany
wms-site.com
The Potential of Mitochondrial Genome Engineering
 Dr. Pedro Silva-Pinheiro, from the University of Cambridge, UK, will join us this year to present his most recent findings on "The Potential of Mitochondrial Genome Engineering".
Dr. Pedro Silva-Pinheiro, from the University of Cambridge, UK, will join us this year to present his most recent findings on "The Potential of Mitochondrial Genome Engineering".
Mitochondria are organelles that host key metabolic processes vital for cellular energy provision and are central to cell fate decisions. They are subjected to unique genetic control by both nuclear DNA and their own multi-copy genome - mitochondrial DNA (mtDNA).
Mutations in mtDNA often lead to clinically heterogeneous, maternally inherited diseases that display different organ-specific presentation at any stage of life. Despite rapid advances in nuclear genome engineering, for years, mammalian mtDNA has remained resistant to genetic manipulation, hampering our ability to understand the mechanisms that underpin mitochondrial disease.
Dr. Pinheiro will discuss the recent developments in the genetic modification of mammalian mtDNA and how these raise the possibility of using genome editing technologies, such as programmable nucleases and base editors, for the treatment of hereditary mitochondrial disease.
Reserve your spot now for Targeting Mitochondria 2022 to attend Dr. Pinheiro 's fascinating talk.
More about Dr. Pinheiro:
Pedro Silva-Pinheiro is a Research Asociate at the MRC Mitochondrial Biology Unit in Cambridge, UK working with Michal Minczuk. He received his BS in Genetics and Biotechnology and a MSc in Molecular Medicine from the University of Porto, Portugal. During his MSc, Silva-Pinheiro was awarded a Fulbright Scholarship allowing to carry his research at the University of Alabama in Birmingham, US, studying the effects of mitochondrial DNA mutations in lipid metabolism. Then, he joined the group of Massimo Zeviani in the MRC Mitochondrial Biology Unit, University of Cambridge supported by a Marie Skłodowska-Curie PhD studentship. During his PhD, Silva-Pinheiro focused on the pathogenesis and development of therapies for mitochondrial diseases using mouse models. Currently, Silva-Pinheiro’s research interests lie on the development and application of new methods for manipulation of the mitochondrial DNA (TALE nucleases and base editors), allowing for the generation of new models of mitochondrial disease and novel therapeutic platforms.
Targeting Mitochondria 2022 Congress
October 26-28, 2022 - Berlin, Germany
wms-site.com
GM1 oligosaccharide as mitochondrial modulator: implications in neurological diseases
 Dr. Maria Fazzari, from the University of Milano, Italy, will join us this year to present her most recent findings on "GM1 oligosaccharide as mitochondrial modulator: implications in neurological diseases".
Dr. Maria Fazzari, from the University of Milano, Italy, will join us this year to present her most recent findings on "GM1 oligosaccharide as mitochondrial modulator: implications in neurological diseases".
Functional data and clinical studies suggest the existence of a positive loop between age-dependent GM1 deficiency and neurodegeneration onset of sporadic Parkinson Disease (PD). This loop is triggered by plasma membrane GM1 deficiency, which leads to a failure of trophic signalling and αS accumulation, increasing the susceptibility to neuronal death. Indeed, GM1 is a ganglioside that has been described as a master regulator of neuronal homeostasis and development, acting through diverse mechanisms including modulation of neurotrophin factors (Trk/RET receptors), Ca2+ signalling, and inhibition of inflammation, excitotoxicity, and oxidative stress.
Mitochondria (mit) dysfunctions have long been implicated in PD etiopathogenesis. Considering the role of mitochondria in energy metabolism, Ca2+ homeostasis and cell death regulation, it is reasonable to speculate that altered mitochondrial function may contribute to the vulnerability of dopaminergic neurons. Accordingly, for decades, MPTP has been one of the most used PD models; it is a neurotoxin exerting its effect by the inhibition of NADH:ubiquinone oxidoreductase, leading to mitochondria energy deprivation, oxidative stress increase and finally to cell death.
Recently, Dr. Fazzari and her team demonstrated that the hydrophilic oligosaccharide of GM1 (GM1-OS) is the bioactive portion of GM1 that, by interacting with the NGF TrkA complex, triggers crucial intracellular pathways responsible for neuronal differentiation, protection and restoration, including antioxidant mechanism and mitochondrial bioenergetics.
Targeting Mitochondria 2022 Congress
October 26-28, 2022 - Berlin, Germany
wms-site.com
A Novel PTD-mediated IVT-mRNA delivery platform developed for Protein Replacement Therapy (PRT) for genetic/metabolic disorders: the case of human fatal infantile cardioencephalomyopathy attributed to SCO2 mutations

Lefkothea C. Papadopoulou, Professor of Pharmacology, from Aristotle University of Thessaloniki, Greece, will join us this year to present her most recent findings on "A Novel PTD-mediated IVT-mRNA delivery platform developed for Protein Replacement Therapy (PRT) for genetic/metabolic disorders: the case of human fatal infantile cardioencephalomyopathy attributed to SCO2 mutations".
Mutations in human SCO2 gene, encoding the mitochondrial inner membrane Sco2 COX assembly protein, have been implicated in the mitochondrial disorder fatal infantile cardioencephalomyopathy accompanied by cytochrome c oxidase (COX) deficiency as well in neurodegenerative disorders, like Leigh syndrome.
Since there is no effective treatment for such disorders yet, a Protein Replacement Therapeutic (PRT) approach via the Protein Transduction domain (PTD) Technology was explored. Protein Transduction Domains (PTDs) or Cell Penetrating Peptides (CPPs), such as TAT, are small peptides, able to facilitate intracellular delivery of a variety of cargos, ranging from small molecules to macromolecules, and permit their localization in subcellular organelles, like mitochondria. In the frame of this work, Dr. Papadopoulou's group cloned the coding sequences of human SCO2 gene and expressed it in bacteria to produce the recombinant human TAT-L-Sco2 fusion protein.
Purified bacterial inclusion bodies, enriched in the fusion protein, were solubilized in 1M L-Arg solution. Exogenously administered TAT-L-Sco2 fusion protein was transduced successfully inside the mitochondria, where it was processed by MPP to yield the mature Sco2 protein, thus contributing to COX assembly and recovery of COX activity in fibroblasts derived from a SCO2/COX deficient patient. Radiolabeled TAT-L-Sco2 fusion protein, prepared and administered in animals (mice), was found to be biodistributed in peripheral tissues and successfully transduced into mitochondria in vivo.
Since the production of recombinant protein therapeutics requires complex purification procedures, increasing the cost of good manufacturing practice (GMP)’s protein products, Dr. Papadopoulou's group moved on to an alternative PRT approach, the PTD-mediated transduction of the vitro transcribed (IVT)-mRNAs. IVT-mRNA, produced in a cell-free system, resembles natural mRNA and its successful intracellular delivery permits transient expression / translation into the protein of interest.
The IVT-mRNA Technology platform has been optimized, in terms of IVT-mRNA structural stability, sensitivity to enzymatic degradation, inadequate translation and immunogenicity. Several structural and chemical modifications in IVT-mRNA increased the amount of protein produced per molecule of delivered mRNA, while reduced its immunogenicity. Furthermore, the potential clinical applications of the IVT-mRNAs strongly depend on their successful delivery to produce enough of the desired protein intracellularly. A wide range of in vitro and in vivo transfection reagents have been shown to facilitate IVT-mRNAs intracellular uptake, including protection from degradation.
Having all these in mind, Dr. Papadopoulou's group developed a novel PTD-mediated IVT-mRNA delivery platform [International Patent Pending, PCT/GR2020/000059; Greek patent: 1010063 (National)] as a protein therapy approach for metabolic / genetic disorders, using the amphipathic PTD, PFVYLI. The selected PTD is exploited as a carrier for any therapeutic IVT-mRNA through a novel, covalent reaction, with puromycin (conjugated via an amide bond to the PTD) served as a linker to the IVT-mRNA. PTD was successfully conjugated to IVT-mRNA of SCO2, as confirmed by NMR data analysis and a band shift assay compared to the corresponding naked IVT-mRNA.
The proposed PTD-IVT-mRNA delivery platform showed significant stability of the studied IVT-mRNA in various conditions, indicating that the PTD protects the IVT-mRNA from immediate nuclease digestion. PTD-IVT-mRNA of SCO2 was successfully transduced (via clathrin-mediated endocytosis) into fibroblasts derived from a SCO2/COX deficient patient and led to the recovery of reduced COX activity, compared to the naked IVT-mRNA as well the IVT-mRNA conjugated to a non-PTD, Control peptide.
These findings suggest that this novel PTD-IVT-mRNA delivery strategy could be a promising platform for effective gene expression/translation into a protein of interest, with the potential to be used in clinical trials as a PRT for metabolic/genetic disorders.
Targeting Mitochondria 2022 Congress
October 26-28, 2022 - Berlin, Germany
wms-site.com
EV-Mitochondria as a Promising Therapeutic for Mitochondrial Dysfunction
 Dr. Devika S Manickam, from Duquesne University, USA will join us this year to present her most recent findings on "EV-Mitochondria as a Promising Therapeutic for Mitochondrial Dysfunction" in a special session dedicated to "Extracellular Vesicles & Mitochondria: The Target" .
Dr. Devika S Manickam, from Duquesne University, USA will join us this year to present her most recent findings on "EV-Mitochondria as a Promising Therapeutic for Mitochondrial Dysfunction" in a special session dedicated to "Extracellular Vesicles & Mitochondria: The Target" .
Extracellular vesicles (EVs) are natural, cell-secreted nanoparticles that play an important role in intercellular communication. Dr. Manickam's work has demonstrated that the innate mitochondrial load in EVs can be used to increase the cellular energetics and mitochondrial function of the recipient brain endothelial cells. She has also demonstrated that the EV mitochondrial cargo can be transferred mouse brain slice neurons. She is currently engineering EVs with a greater mitochondrial load via pharmacological activation of the donor cells.
Dr. Manickam's talk will describe how the EV mitochondrial load can serve as a therapeutic modality to treat neurodegenerative diseases.
Remember, you can share abstracts related to this special session and get the chance to participate in Targeting Mitochondria 2022.
Targeting Mitochondria 2022 Congress
October 26-28, 2022 - Berlin, Germany
wms-site.com
Unlocking the potential of the mammalian electron transport chain
 Dr. Jessica Spinelli, from the Whitehead Institute for Biomedical Research, USA will join us this year to present her most recent findings on "Unlocking the potential of the mammalian electron transport chain".
Dr. Jessica Spinelli, from the Whitehead Institute for Biomedical Research, USA will join us this year to present her most recent findings on "Unlocking the potential of the mammalian electron transport chain".
The continuous flow of electrons in the mitochondrial electron transport chain (ETC) is required for an array of mammalian biosynthetic and bioenergetic pathways. Removal of electrons onto a terminal electron acceptor (TEA), which is known to be oxygen in mammalian cells, maintains continuous electron flow in the ETC. Paradoxically, in low oxygen environments, mammalian cells still catalyze reactions that necessitate electron flow in the ETC, suggesting the existence of alternative mechanisms to remove ETC electrons beyond oxygen reduction.
Here, Dr. Spinelli uncovers a secondary TEA that sustains electron flow in the mammalian ETC upon hypoxia exposure. In mice, the ability to use this adaptive electron removal pathway is tissue-specific and dependent on the presence of a previously uncharacterized mammalian metabolite.
Targeting Mitochondria 2022 Congress
October 26-28, 2022 - Berlin, Germany
wms-site.com
Mitochondrial responses to a massive trauma-determinant of survival
 Dr. Marc G. Jeschke, from the University of Toronto, Canada will join us this year to present his most recent findings on "Mitochondrial responses to a massive trauma-determinant of survival".
Dr. Marc G. Jeschke, from the University of Toronto, Canada will join us this year to present his most recent findings on "Mitochondrial responses to a massive trauma-determinant of survival".
He will be introducing us to the mitochondrial function and associate metabolic changes in a model of severe trauma. He will also delineate changes associated with age.
Targeting Mitochondria 2022 Congress
October 26-28, 2022 - Berlin, Germany
www.targeting-mitochondria.com
2-Deoxy-D-glucose couples mitochondrial DNA replication with mitochondrial fitness
 Dr. Ian J. Holt, from the Biodonostia Health Research Institute, Spain will join us this year to present his most recent findings on "2-Deoxy-D-glucose couples mitochondrial DNA replication with mitochondrial fitness".
Dr. Ian J. Holt, from the Biodonostia Health Research Institute, Spain will join us this year to present his most recent findings on "2-Deoxy-D-glucose couples mitochondrial DNA replication with mitochondrial fitness".
Ever since mutant mitochondrial DNA was discovered to cause human diseases researchers have sought to understand how defective mitochondria evade natural selection, and to find ways to combat them. Most mutant mitochondrial DNA variants cause disease only when they account for substantially more than half the total population of molecules, and so decreasing the level of the mutants by a small amount can produce dramatic improvements in mitochondrial function.
Dr. Holt discovered that the replication of mutant mitochondrial DNA is equal to that of wild-type molecules when nutrients are plentiful; however, nutrient restriction inhibits the replication of mutant mtDNA. Using small molecules that limit nutrient consumption he then decreased the mutant load in cells of patients with mitochondrial DNA disease.
These findings indicate the parasitic nature of mitochondria with mutant mtDNA and identify a highly promising therapeutic strategy that we are advancing towards the clinic.
Targeting Mitochondria 2022 Congress
October 26-28, 2022 - Berlin, Germany
www.targeting-mitochondria.com
Mitochondrial Transfer via MitoPunch

Dr. Michael Teitell, from the University of California, USA will join us this year to present his most recent findings on "Mitochondrial Transfer via MitoPunch".
He will be discussing the use of MitoPunch to deliver mitochondria containing mitochondrial DNA (mtDNA) into cells lacking mtDNA.
Targeting Mitochondria 2022 Congress
October 26-28, 2022 - Berlin, Germany
www.targeting-mitochondria.com